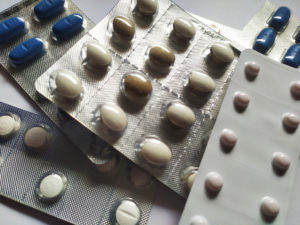
On October 11, 2016, homeopathic medicine manufacturer Hyland’s issued a public letter to parents everywhere. Beginning with “Dear Moms and Dads…” the corporate communication went on to say that they would no longer be distributing their line of homeopathic teething tablets and gels in the U.S. They also admitted in this letter that the decision was made in light of a recent (Sep. 2016) warning issued by the Food & Drug Administration (FDA) against the categorical use of homeopathic teething tablets and gels.
The letter goes on to say that, while many retailers chose to pull such products from their shelves (again, categorically), there have been some who continue to sell the teething products—but this is nothing to worry about, because Hyland’s products are safe and have been for over 100 years.
What the letter does not tell you is that, this is not the first time the Los Angeles-based company has had issue with the FDA. In fact, the government agency’s initial interest in the homeopathic teething product goes back over a decade. From 2006 to 2016, the FDA was notified of “adverse events” involving more than 370 children who had used Hyland’s homeopathic teething tablets or gel, with reported effects including seizures, delirium, tremors and uncontrollable twitching—there are even eight infant deaths still under review by the agency.
So why did it take so long for such a questionable product to be discontinued? Mainly, the issue was that the FDA has no authority to enforce a recall of homeopathic products. Sure, they pushed the company to recall and reformulate the product in 2010. And they issued a request for another recall in January of 2017 that went unheeded. Yet the fact is that even after discontinuation, the product is still out there and easily purchased from independent and online retailers.
Alternatively, the American Academy of Pediatrics suggests that parents might try giving the teething child a clean, wet washcloth that’s been chilled in the freezer or even frozen fruit or bagel pieces to chew on. Ultimately, the advice to any parent is to be careful about any treatment you might purchase for your child—and maybe question if a medication is really needed at all.

The Legal Examiner and our Affiliate Network strive to be the place you look to for news, context, and more, wherever your life intersects with the law.
















2 Comments
ChristyRedd
It's hard to see how a product which contains two trillionths of a gram of substance could possibly cause harm. The FDA analysis of Hyland's teething tablets showed that, at most, some tablets contained 0.1 to 53.4 nano-grams of substance; that is, 0.1 to 53.4 billionths of a gram.
Compare that to Donnata, which the FDA approves for sale, which contains 0.0065 milligrams of scopolamine, 0.0194 milligrams of atropine sulfate and 0.1037 milligrams of hyoscamine sulfate.
Sandra
No other country's drug enforcement agency has made such a claim against teething tablets containing belladonna. It's bogus.
Comments for this article are closed.